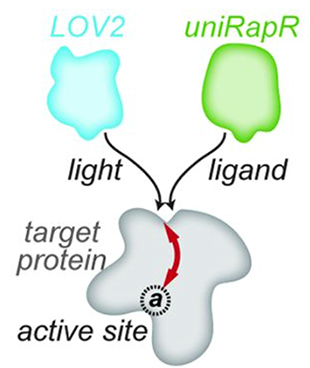
Domains conferring either photo-inhibition (LOV2) or activation induced by small molecules (uniRapR) are inserted at allosteric sites away from the active sites. The "loopology" approach identifies "tight loops" on the surface that are allosterically coupled to the active site, and that can be controled through insertion of engineered domains.
The light-induced conformational change of the LOV domain is rapid (< 0.5 milliseconds). The half time for return to the dark state can be adjusted using point mutations (in our hands from 1.7 to 496 seconds, Curr. Protoc. Cell Biol., 73: 21.10.1-14, 2016). Rapid return times are useful for precise kinetic control. Slower return times enable continuous activation with only a brief pulse of light every few minutes.
Rreferences
Dagliyan, O., Tarnawski, M., Chu, P-H., Shirvanyants, D., Schlichting, I., *Dokholyan, N.V., and *Hahn, K.M. Engineering extrinsic disorder to control protein activity in living cells. Science, 354(6318): 1441-1444, 2016. PMC5362825. View free in Science online: Full text & PDF | Free PMC article
Methods articles
Dagliyan, O., Dokholyan, N.V., and Hahn, K.M. Engineering proteins for allosteric control by light or ligands. Nature Protocols, 14(6): 1863-1883, 2019. PMC6648709. Online article | Free PMC article available 12/01/2019
Karginov, A.V. and Hahn, K.M. Allosteric activation of kinases: design and application of RapR kinases. Curr. Protoc. Cell Biol., 53: 14.13.1 – 14.13.16, 2011. PMC3269071. Online article | Free PMC article
~ Updated 04/08/2021